A fascinating idea.
From New Atlas
Aluminum has an energy density more than 50 times higher than lithium ion, if you treat it as an energy storage medium in a redox cycle battery. Swiss scientists are developing the technology as a renewable energy stash for the European winter.
The problem is simple enough: as countries worldwide plan their moves toward zero-emissions energy, they need to deal with the intermittent nature of cheap renewable energy. On a daily basis, solar harvests most of its energy in the middle of the day, and this necessitates some kind of short-term storage solution that can park that energy in some form of battery, then release it again in the evening when everyone gets home and starts running TVs and dishwashers. These kinds of big battery projects are already installed in many areas and proving their worth.
But intermittency is a much bigger issue on a seasonal level. The further you move from the equator, the less Sun you get in the winter months. Parts of Scandinavia famously get no Sun at all for months on end – resulting in some pretty epic springtime parties, I'm told – but a much broader area is going to find itself very short on solar, every year, right when everyone's starting to crank up their heaters. The zero-carbon world needs a way to store absolutely massive amounts of excess renewable energy generated in the warmer months, then release it through the long winters. And it'll need to be affordable, or else it's not going to happen.
Researchers from Switzerland's SPF Institute for Solar Technology have been studying aluminum redox cycles for many years now, and with funding from the EU's Horizon Europe program and the Swiss government, they've just kicked off a research project called Reveal, drawing in nine different partners from seven European countries, to develop what looks like a very promising idea.
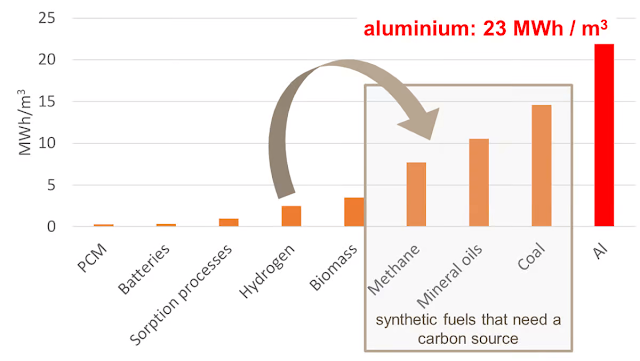
As a 2020 report from the SPF team states, a single, one cubic meter (35.3 cu ft) block of aluminum can chemically store a remarkable amount of energy – some 23.5 megawatt-hours, more than 50 times what a good lithium-ion setup can do, or roughly enough to power the average US home for 2.2 years, on 2020 figures. That's by volume – going by weight, aluminum holds a specific energy of 8.7 kWh per kilogram, or about 33 times more than the batteries Tesla uses in its Model 3.
Big fat blocks like that aren't exactly practical to work with, though, so the Reveal team proposes using 1-mm (0.04 in)-diameter balls of aluminum instead. Naturally, you lose some volumetric density here, but you're still coming out over 15 MWh per cubic meter.
 |
SPF Institute for Solar Technology |
Getting that energy in and out is, of course, a lot more involved. During the "charging process," excess renewable energy would be used to convert aluminum oxide, or aluminum hydroxide, into pure, elemental aluminum. This is an industrial electrolysis process, requiring temperatures around 800 °C (1,472 °F), as well as novel inert electrodes, if you want to avoid the carbon dioxide emissions that accompany today's conventional aluminum smelting processes.
The team estimates it'll be possible to "charge" an aluminum redox system like this at an efficiency around 65%. All the raw materials here are relatively cheap and abundant, some of them indeed being scrap, with the added benefits of being very simple to store and transport. Yes, aluminum oxidizes on contact with ambient air, but it's only a surface layer, less than half a nanometer thick, representing a chemical energy loss of "far less than 1%" when those tiny 1-mm balls are stored in air.
To discharge the aluminum, you simply convert it back again. This can be done at low temperatures, using aluminum-water reactions at less than 100 °C (212 °F), generating aluminum hydroxide, along with pure hydrogen, which can be run straight into a PEM fuel cell stack for conversion to electricity. The process and the fuel cell also generate heat, which can be recovered at temperatures relevant for space heating or domestic hot water.
 |
SPF Institute for Solar Technology |
There's also a higher-temperature process, running at over 200 °C (392 °F), which reacts the aluminum with steam to generate aluminum oxide, hydrogen and much higher levels of heat, more relevant for industrial applications.
In the Reveal model, the charging process would be done at central smelting depots, and the "charged-up" aluminum would be trucked out in bulk to be "discharged" on-site at apartment buildings, industrial facilities, and even individual homes, since the equipment needed is relatively simple and low-maintenance – well, apart from the fact that the aluminum-to-hydrogen conversion system doesn't exactly exist yet at this point.
Once it's out of juice, the aluminum oxides and hydroxides would be sent back to the depot for "recharging." Ideally, the Reveal team says, this aluminum will be cycled back and forth in this process indefinitely, so there won't be any ongoing raw material costs for a given system.

No comments:
Post a Comment